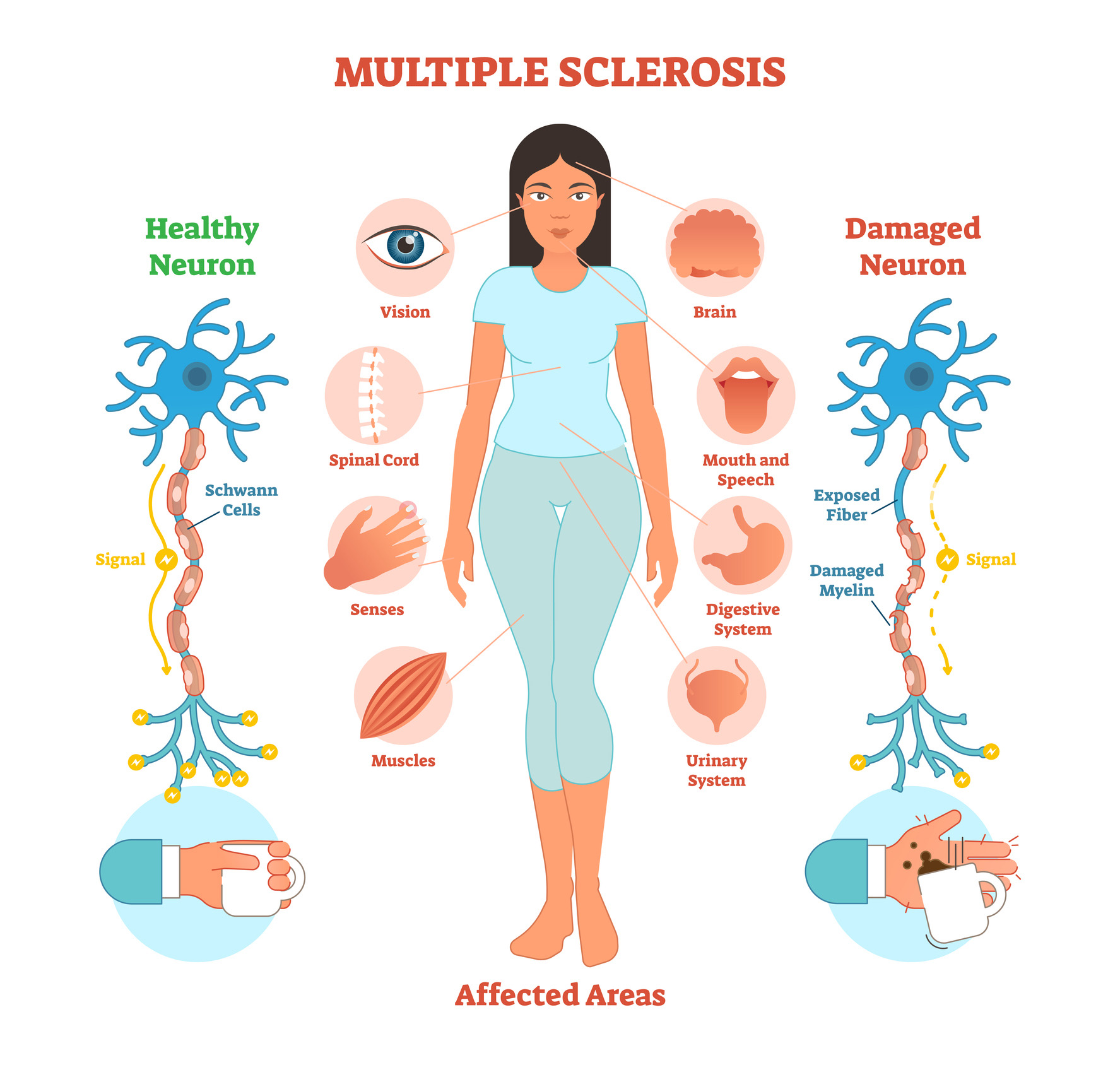
Multiple Sclerosis (MS) is a chronic neurodegenerative disease that has affected the lives of 2.3 million individuals worldwide, as of 2022. In the United States, MS affects a little over one million people, and it is the leading cause of non-traumatic neurological disability in young adults. The pathogenesis of MS reveals that it is an immune-mediated demyelinating disorder in which the immune system misidentifies myelin in the central nervous system as a foreign object and attempts to destroy it. Furthermore, the disease is characterized by oligodendrocyte cell loss which prevents the possibility of remyelination. The disease's symptoms are not universal, as many people have had various neurological deficits. There are two types of MS: relapse-form and progressive-form. Most MS patients will experience Relapsing-Remitting MS (RRMS), which is characterized by neurological deficits for a period of time followed by recovery (“Introduction to MS - Progression” 2022). The duration of neurological symptoms varies between individuals. A majority of RRMS patients will enter another disease phase during the disease progression called Secondary-Progressive MS (SPMS) (“Introduction to MS - Progression” 2022). SPMS is characterized by increasing (irreversible) neurological deficits unrelated to relapses and a worsening of health from onset. Of those patients who have SPMS, 10 percent will have Primary-Progressive MS (PPMS) where they never have disease “attacks” (“Introduction to MS - Progression” 2022). Axonal degeneration is primarily responsible for the irreversible neurological functional decline in most MS patients. There is no cure for this disease at the moment, but disease-modifying therapies (DMTs) are widely used to treat MS patients. The current treatments address the symptoms, attempt to reduce relapses, and postpone disease progression.
During Dr. Yanan Chen’s talk on Sephin1, Which Prolongs the Integrated Stress Response, is a Promising Therapeutic for Multiple Sclerosis, she had mentioned that the oligodendrocyte-protective nature of the molecule Sephin1 could be utilized as a potential therapeutic to slow down disease progression. Currently, there are only 15 FDA-approved DMTs for RRMS; however, patients with SPMS have only three options (Collazo 2022). Patients with PPMS now have an option as the newest medication for PPMS and RRMS was approved by the FDA in 2017. This DMT has been on the market for around five years, and so Dr. Yvette N. Lamb investigated therapeutic efficacy and the safety of Ocrelizumab (Ocrevus®) on PPMS and RRMS patients (Lamb 2022).
Ocrelizumab, sold in the market under the brand name of Ocrevus, “humanized anti-CD20 monoclonal antibody” which is administered intravenously in patients who qualify (Lamb 2022). The medication is attempting to achieve the eradication of three clinical symptoms of MS. The first goal is to delay the clinical progression in patients with PPMS (Lamb 2022). The second goal is to reduce relapses, delay disease progression, and reduce inflammatory responses in RRMS patients (Lamb 2022). The third goal is to maintain the same clinical therapeutic efficacy even after long-term treatment use (Lamb 2022).
In order to test the therapeutic efficacy, Dr. Lamb designed two separate experiments, one for RRMS patients and the other for PPMS. In a randomized, and double-blind experimental design, RRMS patients either received 600 mg infusions of Ocrelizumab or subcutaneous interferon β-1a 44 µg for 96 weeks (Lamb 2022). To establish a baseline, each individual took a test to determine where they were on the Expanded Disability Status Scale (EDSS), an MRI scan to ensure brain abnormalities are consistent with those of RRMS patients and no neurological deficits in the 30 day range prior to the experiment. Results from this experiment revealed that Ocrelizumab had “significantly reduced the annualized relapse rate” in comparison to the subcutaneous interferon β-1a 44 µg (Lamb 2022). In addition, Ocrelizumab also significantly improved disabilities as scores of the EDSS were lower than baseline. Also there was little change in the MRI scans from baseline (Lamb 2022). After long term use of the treatment delivered intravenously, the tolerability and safety of the medication was determined through observations. It was seen as tolerable and safe as the side effects of both Ocrelizumab and subcutaneous interferon β-1a 44 µg were at the same levels indicating that the DMT was tolerable and safe for long-term usage (Lamb 2022). RRMS patients did suffer from mild “infections such as upper respiratory tract infection, nasopharyngitis and urinary tract infections” during the initial use of Ocrelizumab but adjusted easily in later infusion treatments (Lamb 2022).
In a randomized, and double-blind experimental design, PPMS patients either received 600 mg infusions of Ocrelizumab or a matching placebo every 24 weeks for 120 weeks (Lamb 2022). To establish a baseline, each individual took a test to determine where they were on the Expanded Disability Status Scale (EDSS), Functional Systems Scale test and IgG index test and PPMS patients must have had MS symptoms for less than 15 years prior to the experiment. Results from this experiment revealed that Ocrelizumab had efficacy in delaying the clinical progression of PPMS in comparison to the placebo (Lamb 2022). The tolerance and side effects seen were the same for both Ocrelizumab and the placebo indicating that Ocrelizumab is well tolerated and safe after 120 weeks of intravenous deliverance. Furthermore, there was little to none in PPMS patients who had progression or treatment discontinuation due to an adverse infusion-related reaction (Lamb 2022).
This medication can be utilized by patients who have an active RRMS or patients who have early PPMS (Lamb 2022). Although the exact underlying biological mechanism of ocrelizumab's therapeutic benefits in MS patients is unknown, further research could provide more insights into a potential cure for PPMS and RRMS. People's lives are at stake, which should motivate us neuroscientists to conduct more diligent research into this incurable disease.
References
Collazo, Dr. Irin M. "Emerging Treatments for Multiple Sclerosis." Mayo Clinic, 14 July
2022, https://www.mayoclinic.org/diseases-conditions/multiple-sclerosis/expert-
answers/emerging-treatments-for-ms/faq-20096786. Accessed on 1 Dec 2022.
"Introduction to MS - Progression." My MS, 2022, https://my-ms.org/ms_progression.htm.
Accessed on 1 Dec 2022.
Lamb, Yvette N. "Ocrelizumab: A Review in Multiple Sclerosis." Drugs, vol. 82, 22 Feb 2022,
pp. 323-334, https://doi.org/10.1007/s40265-022-01672-9. Accessed on 1 Dec 2022.


No comments:
Post a Comment